This forum is intended for questions about kinetics, Surface Plasmon Resonance and the instruments related to these techniques.
Integrated rate equation for association
- Drug discovery
- Topic Author
- Offline
- New Member
-

Less
More
- Posts: 2
- Thank you received: 0
1 year 4 months ago #1
by Drug discovery
Integrated rate equation for association was created by Drug discovery
Please Log in or Create an account to join the conversation.
- Arnoud
-

- Offline
- Moderator
-

Less
More
- Posts: 45
- Thank you received: 5
1 year 4 months ago #2
by Arnoud
Replied by Arnoud on topic Integrated rate equation for association
Please Log in or Create an account to join the conversation.
- Drug discovery
- Topic Author
- Offline
- New Member
-

Less
More
- Posts: 2
- Thank you received: 0
1 year 4 months ago #3
by Drug discovery
Replied by Drug discovery on topic Integrated rate equation for association
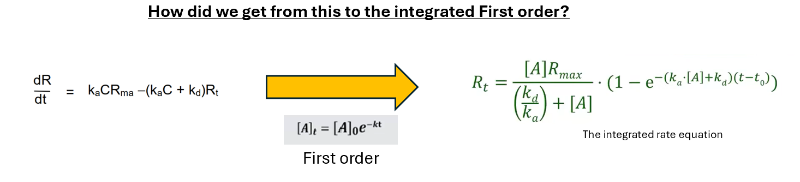
Thank you Arnoud. I got the steps before and after integration but not the actual integration step.
I was keen to how the variables were separated from the previous step and the subsuequent integration to end up with Ka.C.Rmax / (kaC+Kd)x(1-e*(kaC+Kd)t Just couldn't figure.. yet
Thank you as always for the quick repsonse and amazing contirbution!!
cheers
I was keen to how the variables were separated from the previous step and the subsuequent integration to end up with Ka.C.Rmax / (kaC+Kd)x(1-e*(kaC+Kd)t Just couldn't figure.. yet
Thank you as always for the quick repsonse and amazing contirbution!!
cheers
Please Log in or Create an account to join the conversation.
- thomeseliana
- Offline
- New Member
-

Less
More
- Posts: 3
- Thank you received: 0
1 year 3 months ago #4
by thomeseliana
Replied by thomeseliana on topic Integrated rate equation for association
When discussing the integrated rate equation for association, it’s essential to understand that it describes the time-dependent formation of a complex between two molecules (like a ligand and a receptor). The equation relates the concentration of the complex to the rate constants for association and dissociation. [Deleted]
Visit here: [Deleted]
Visit here: [Deleted]
Please Log in or Create an account to join the conversation.
- NMCCANN2
- Offline
- New Member
-

Less
More
- Posts: 12
- Thank you received: 2
1 year 3 months ago #5
by NMCCANN2
Replied by NMCCANN2 on topic Integrated rate equation for association
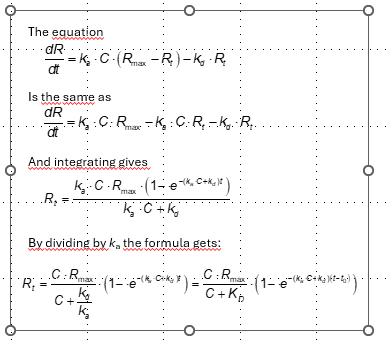
I've tried to go through this derivation myself and it is not straight forward. There is a lot of strange algebraic manipulation to get there. I was only able to solve it by starting with the integrated rate equation and working backwards toward the differential equation.
Please Log in or Create an account to join the conversation.
- NMCCANN2
- Offline
- New Member
-

Less
More
- Posts: 12
- Thank you received: 2
1 year 3 months ago #6
by NMCCANN2
Replied by NMCCANN2 on topic Integrated rate equation for association
Here, I drew out the differentiation from the integrated form to the differential form.
Please Log in or Create an account to join the conversation.
Moderators: Arnoud