Sensor chip NTA - nitrilotriacetic acid
Sensor chip NTA (NTA: nitrilotriacetic acid) is designed to bind histidine-tagged molecules.
The binding of the histidines relies on a NTA-chelated nickel atom. The affinity (KD ≅ 10-6 M (1) of this interaction is commonly sufficiently high to allow detailed analysis of subsequent analyte binding. Immobilization via His-tags has also the advantage of orientating the ligand molecules in a homogeneous way and allowing the immobilization to be carried out without significant changing the pH or ionic strength during the coupling procedure.
However, as with many other affinity tags (e.g. biotin and antigen epitopes) the affinity may vary with the microenvironment created by moieties adjacent to the His-tag. The affinity can also be affected by the buffer environment, e.g. pH and ionic strength. The Sensor chip NTA is more sensitive for changes in conditions than the NTA affinity columns (2),(3). Side chains of cysteine, tyrosine, tryptophan and lysine on the surface of a protein may participate in binding to a chelated metal. Although the affinity of these interactions are typically significantly lower than that commonly obtained with histidine tags.
Suitable ligand concentrations are typically below 200 nM. Higher ligand concentrations may produce complex binding curves and less stable binding of the ligand when "non-his" sites with low affinity begin to participate in binding to the nickel atom. For the best results, the histidine-tagged ligand should be purified prior to immobilization and prepared in eluent buffer. Ligand concentration and contact time, typically in the range 1-15 minutes, are used to control the amount of bound ligand. Low flow rates (2-5 µl/minute) can be used to achieve long contact times.
In some cases, it is possible to dissociate the bound His-protein with Ni2+. It is thought that the free Ni2+ will compete for the immobilized Ni2+ and so for the bound His-protein.
EDTA in the buffers
The use of EDTA in the eluent buffer helps to neutralize contaminating metal ions that may be present in compounds used to prepare the buffer, but is diluted enough not to strip the nickel from the surface. The use of two buffers is to avoid binding to free metal ions to the his-tagged proteins (1),(3).
Some non-specific binding of crude ligand samples may be expected due to binding to the Ni2+(1). Adding 250 µM EDTA to the ligand sample may reduce non-specific binding.
O'Shannessey (2) tested three buffers (Tris, Hepes, phosphate) and concluded that Hepes gives the best reproducible and sensitive results. Adding 8 M urea to the samples is possible without affecting the binding.
| Buffer | Solution |
|---|---|
| Eluent buffer (eluent pump) | 10 mM HEPES, 150 mM NaCl, 50 µM EDTA. 0.005% Surfactant P20, pH 7.4 |
| Dispensor buffer (dispensor pump) | 10 mM HEPES, 150 mM NaCl, 3 mM EDTA, 0.005% Surfactant P20, pH 7.4 |
| Nickel solution | 500 µM NiCl 2 in eluent buffer or 1 mM NiNO 3 in 50 mM Tris pH 7.5 |
| Regeneration solution | 10 mM HEPES, 150 mM NaCl, 350 mM EDTA, 0.005% Surfactant P20, pH 8.3 or 50 mM Imidazole |
Example of NTA sensor chip
First step is loading the sensor chip with NiCl2 (20 µl of 500 µM with 20 µl/min should give a baseline rise of ± 40 RU), followed by an injection with a His-tagged protein and subsequently by dissociation. The injection with EDTA (30 µl at 10 µl/min) strips all the His-tagged protein and Nickel, which is demonstrated by the lack of binding after injection with the same His-tagged protein.
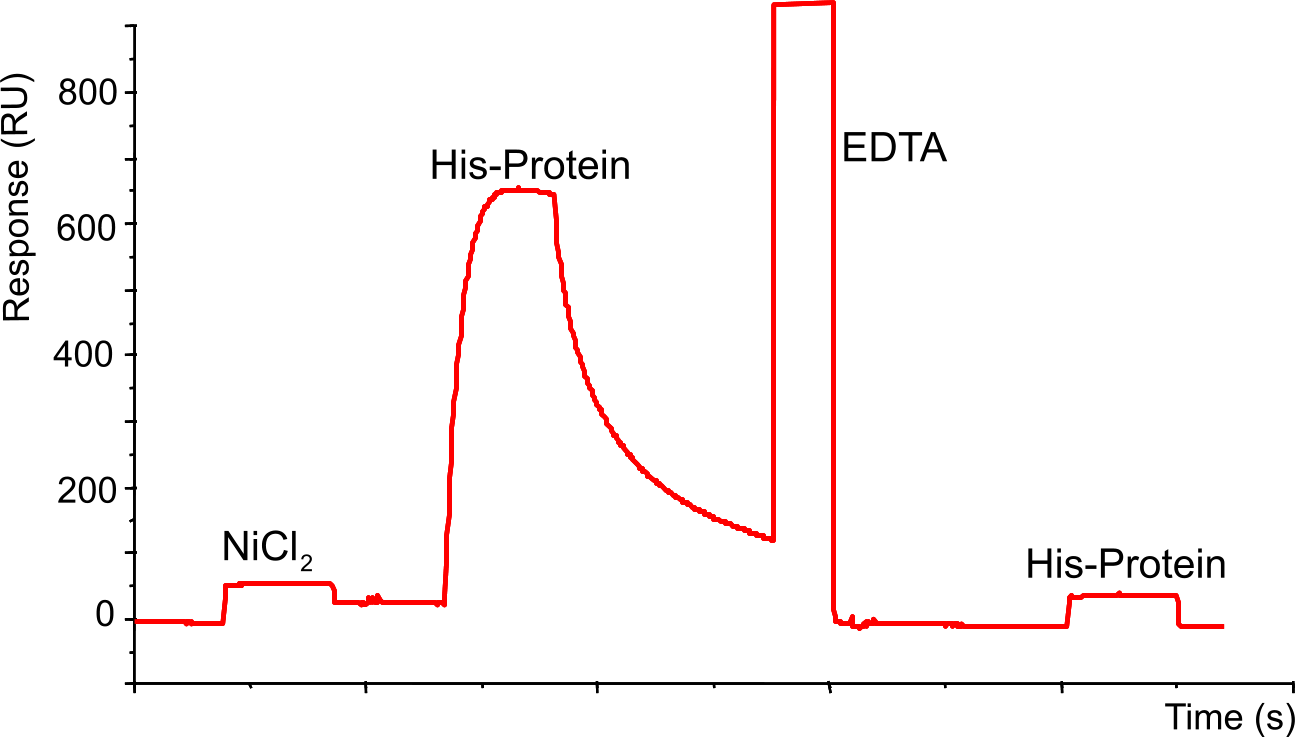
A closer look at the His-protein association reveals that during the injection of the protein, there is a maximum in association followed by a slight dissociation during the injection.
This is explained by rebinding effects (1),(3). Low affinity binding needs continuous rebinding to generate stable binding. Thus, when only a part of the ligand sites are occupied, the binding seems to be more stable than at high concentrations. High concentrations give multiphasic binding curves and a drop in binding levels during injection phase indicating a less stable binding. At high concentration, there are fewer free binding sites thus higher dissociation constants. In addition, at higher flow rates the dissociation rate is increased, which is a further support that rebinding is important in this system.
NTA versus IDA
As chelating agents, both NTA and IDA (iminodiacetic acid) are used (5) in immobilized metal-ion affinity chromatography (IMAC) (6). The difference between these two is that NTA forms a tetra coordinate complex with a metal ion leaving two free coordination sites while IDA forms a tri coordinate complex leaving three free coordination sites.
Hale (5) describes the oriented immobilization of an antibody via the C-terminal part of the heavy chain (in IMAC not sensor chips). After loading the IDA with Co2+ and the binding of the antibody, the Co2+ is oxidized with 0.03% H2O2 to Co3+, which will form an irreversible complex with the bound antibody. After washing with 0.5 M EDTA to remove unoxidized Cobalt and unbound antibody, the surface is ready to use. Afterwards the Cobalt is reduced with 1 M Β-mercaptoethanol to Co2+, which releases the antibody from the system.
References
| (1) | Nieba, L. et al BIACORE analysis of histidine-tagged proteins using a chelating NTA sensor chip. Analytical Biochemistry 252: 217-228; (1997). |
| (2) | O'Shannessy, D. J. et al Detection and quantitation of hexa-histidine-tagged recombinant proteins on western blots and by a surface plasmon resonance biosensor technique. Analytical Biochemistry 229: 119-124; (1995). |
| (3) | Gershon, P. D. and Khilko, S. Stable chelating linkage for reversible immobilization of oligohistidine tagged proteins in the BIAcore surface plasmon resonance detector. Journal of Immunological Methods 183: 65-76; (1995). |
| (4) | Graumann, K. and Jungbauer, A. Quantitative assessment of complex formation of nuclear-receptor accessory proteins. Biochem.J. 345 Pt 3:627-36.: 627-636; (2000). |
| (5) | Hale, J. E. Irreversible, oriented immobilization of antibodies to cobalt-iminodiacetate resin for use as immunoaffinity media. Analytical Biochemistry 231: 46-49; (1995). |
| (6) | Müller, K. M. et al Tandem immobilized metal-ion affinity chromatography/immunoaffinity purification of His-tagged proteins - Evaluation of two anti-His-tag monoclonal antibodies. Analytical Biochemistry 259: 54-61; (1998). |