DNA mediated coupling
This type of immobilization depends on the annealing of two complementary oligonucleotides modified with a tag. Clearly this method is more labor intensive to set up and the ligand has to be conjugated with biotin (1).
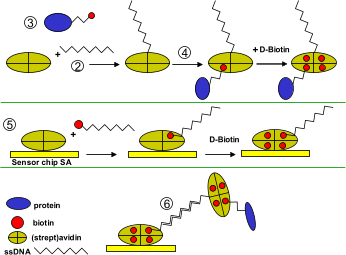
| 1) | Two complementary oligonucleotides are made. One 5'-thiol modified and one 5'-biotin oligonucleotide. |
| 2) | The 5'-thiol oligonucleotide is covalent bound to a recombinant streptavidin to form a DNA-Strp hybrid. |
| 3) | The protein that serves as ligand is biotinylated. |
| 4) | The DNA-streptavidin hybrid is mixed with the biotinylated protein and incubated for 30 minutes at room temperature (20 mM Tris-HCl, 150 mM NaCl, 5 mM EDTA, 0.01% Triton X-100, 0.1 mg/ml BSA, pH 7.5). After dilution a small amount of D-biotin is added and incubated for 10 minutes at room temperature to block free binding places. |
| 5) | On a biacore SA chip the biotinylated oligonucleotide is captured by injecting 10 µM in 30 mM Sodium Phosphate, 150 mM NaCl, 3 mM EDTA, 0.025% Triton X-100, pH 7.4 for 4 minutes. Loosely attached material is removed with three injections of 10 µl 50 mM NaOH. Free biotin binding places are blocked with an injection of D-biotin. |
| 6) | The Ligand-DNA mix is injected over the DNA-surface and allowed to hybridize. Different injection times and concentrations can control the amount of ligand immobilized on the surface. |
| 7) | Regeneration is done by injection of 10 µl 50 mM NaOH, which will denature the DNA bond and release the ligand-streptavidin-DNA hybrid. |