Thiol coupling
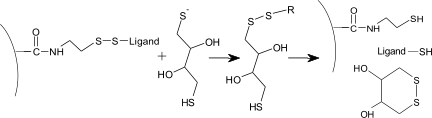
The thiol disulfide exchange coupling can be done in two ways depending on the ligand. Furthermore, the thiol bond can be reduced to strip the sensor chip surface.
In surface thiol coupling the sensor chip surface has thiol groups and the ligand is modified to have active disulfide groups.
In ligand thiol coupling the sensor chip surface has the active disulfide groups and the ligand the free thiol groups.
In reuse of thiol coupling a with a thiol linked ligand immobilized sensor chip is stripped and again immobilized with a thiol method.
Ligand thiol coupling
This procedure activates the sensor chip surface with NHS/EDC and modifies the formed esters with PDEA (2-(2pyridinyldithio)ethaneamine to reactive disulfide groups. Then the ligand is coupled by the reaction of a free -SH group on the ligand.
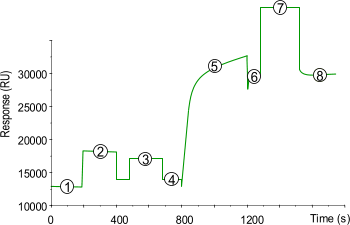
| 1) | Baseline for the unmodified sensor chip surface under continuous flow (5 µl/min). |
| 2) | 10 µl injection of NHS/EDC to activate the surface by modification of the carboxymethyl groups to N-Hydroxysuccinimide esters. |
| 3) | The introduction of a reactive disulfide group with a 20 µl injection of 80 mM PDEA (2-(2-pyridinyldithio)ethaneamine hydrochloride; Mw 222.7) in 0.1 M borate buffer pH 8.5. |
| 4) | Baseline after activation. Activation of the surface has itself only a very slight effect on the SPR signal (100–200 RU). |
| 5) | Injection of ligand (10–200 µg/ml) leads to electrostatic attraction and coupling to the surface matrix. At this point, the ligand solution is still in contact with the sensor surface, and response includes both immobilized and non-covalently bound ligand. The reactive disulfide group react spontaneously with the thiols on the ligand to form covalent links. |
| 6) | Immobilized ligand before deactivation. The ligand has passed the sensor surface and most of the protein that is not covalently bound is eluted. |
| 7) | Deactivation of unreacted disulfide groups with 20 µl 50 mM l-cysteine, 1 M NaCl in 0.1 M formate buffer pH 4.3. The increased SPR signal is due to a change in the bulk refractive index. The deactivation process also removes any remaining electrostatically bound ligand. |
| 8) | Point 8 minus point 4 gives the amount of immobilized ligand after deactivation. |
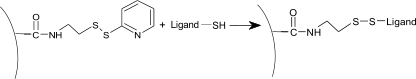
Surface thiol coupling
With surface thiol coupling the ligand is first modified. By introducing a reactive disulfide group it can react with the thiol group on the sensor chip surface.
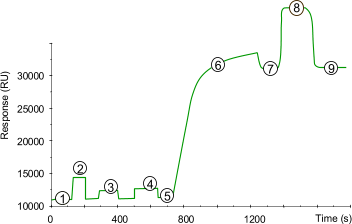
| 1) | Baseline for the unmodified sensor chip surface with continuous flow (5 µl/min). |
| 2) | 15 µll injection of NHS/EDC to activate the surface by modification of the carboxymethyl groups to N-Hydroxysuccinimide esters. |
| 3) | First step in the introduction of thiol group is done with a 15 µll injection of 40 mM cysteamine in 0.1 M borate buffer pH 8.0. |
| 4) | Second step in the introduction of thiol group is done with a 15 µll injection of 0.1 M DTE (Dithioerythritol: erythro-1,4-dimercapto-2,3 butanediol; Mw 154.25) or DTT in 0.1 M borate buffer pH 8.0. |
| 5) | Baseline after activation. Activation of the surface has itself only a very slight effect on the SPR signal (100 - 200 RU). |
| 6) | Injection of ligand (10 - 200 µlg/ml) leads to electrostatic attraction and coupling to the surface matrix. At this point, the ligand solution is still in contact with the sensor surface, and response includes both immobilized and non-covalently bound ligand. The reactive disulfide group on the ligand reacts spontaneously with the thiols on the sensor chip surface to form covalent links. |
| 7) | Immobilized ligand before deactivation. The ligand has passed the sensor surface and most of the protein that is not covalently bound is eluted. |
| 8) | Deactivation of unreacted thiol groups with 20 µl 20 mM PDEA, 1 M NaCl in 0.1 M formate buffer pH 4.3. The increased SPR signal is due to a change in the bulk refractive index. The deactivation process also removes any remaining electrostatically bound ligand. |
| 9) | Point 9 minus point 5 gives the amount of immobilized ligand after deactivation. |
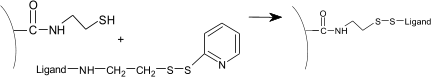
Reuse of thiol coupling
The following method allows the reuse of the sensor chip surface by removing the ligand and reactivates the surface (1).
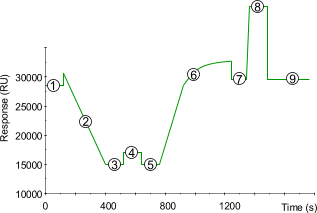
| 1) | Baseline for the unmodified sensor chip surface with continuous flow (5 µl/min). |
| 2) | Removal of the bound ligand by reduction of the disulfide bond. Inject 15 µl of 100 mM DTT in borate buffer. |
| 3) | New baseline after removing the ligand. Wash until stable with HBS. |
| 4) | Reactivation of the surface by binding a thiopropyl group to the exposed cysteamines. Inject 15 µl of PDEA. |
| 5) | Baseline after reactivation. Activation of the surface has itself only a very slight effect on the SPR signal (100 - 200 RU). Wash until stable with HBS. |
| 6) | Injection of ligand (10 - 200 µg/ml) leads to electrostatic attraction and coupling to the surface matrix. At this point, the ligand solution is still in contact with the sensor surface, and response includes both immobilized and non-covalently bound ligand. The reactive disulfide group on the sensor chip surface reacts spontaneously with the thiols on the ligand to form covalent bonds. Wash until stable with HBS. |
| 7) | Immobilized ligand before deactivation. The ligand has passed the sensor surface and most of the protein that is not covalently bound is eluted. |
| 8) | Deactivation of unreacted thiol groups with 20 µl of 50 mM l-cysteine, 1 M NaCl in formate buffer. The increased SPR signal is due to a change in the bulk refractive index. The deactivation process also removes any remaining electrostatically bound ligand. |
| 9) | Point 9 minus point 5 gives the amount of immobilized ligand after deactivation. |
References
| (1) | Cunningham, B. C. and Wells, J. A. Comparison of a structural and a functional epitope. Journal of Molecular Biology 234: 554-563; (1993). |