Interaction kinetics
Analysis of binding experiments is based on a simple model, called the "law of mass action" (1), (2), (3). Binding occurs when analyte (A) and ligand (L) collide due to diffusion, and when the collision has the correct orientation and enough energy, thus forming a complex (LA). Once binding has occurred, the ligand and analyte remain bound together for a random amount of time. After the interaction the components leave each other unchanged as opposed to enzyme kinetics.
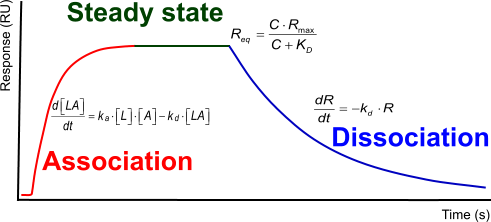
The interaction kinetics can be subdivided in three distinct phases.
- Association: two or more molecules bind to each other
- Steady state: the amount of molecules that is binding is equal to the amount of bonds that is breaking
- Dissociation: the breaking of the bounds between the molecules
Each of the three phases contains information over the interaction between the molecules in terms of how fast the association or dissociation is and how strong the overall interaction.
Regeneration
When the dissociation is very slow, it takes a long time before both components are free. Therefore, a solution with for instance low pH or high salt is injected to break the interaction. This fourth phase is referred to as the regeneration.
References
| (1) | Graphpad Software Graphpad Prism. (1998). Goto reference |
| (2) | Biacore AB BIACORE Technology Handbook. (1994). |
| (3) | Voit, E. O., H. A. Martens and S. W. Omholt 150 Years of the Mass Action Law. PLoS Computational Biology 11: e1004012; (2015). Goto reference |