Aldehyde coupling
Aldehyde coupling may be useful in specific cases. Especially with oxidized glycoproteins the aldehyde coupling is a well established method (1). For more information refer to the BIA Application book of BIACORE (2).
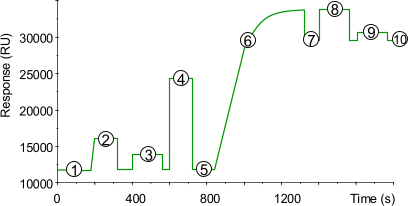
| 1) | Baseline for the unmodified sensor chip surface with continuous flow (5 µl/min). |
| 2) | 15 µl injection of NHS/EDC to activate the surface by modification of the carboxymethyl groups to N-Hydroxysuccinimide esters. |
| 3) | The introduction of a hydrazide group is done with a 35 µl injection of 5 mM hydrazine or carbohydrazine in water. |
| 4) | Next, the unreacted esters are blocked with a 35 µl injection of 1 M ethanolamine pH 8.0. |
| 5) | Baseline after activation. Activation of the surface has itself only a very slight effect on the SPR signal (100–200 RU). |
| 6) | Injection of ligand (10–200 µg/ml) leads to electrostatic attraction and coupling to the surface matrix. At this point, the ligand solution is still in contact with the sensor surface, and response includes both immobilized and non-covalently bound ligand. The reactive hydrazide group on the sensor chip surface reacts spontaneously with the aldehydes on the ligand forming covalent bonds. |
| 7) | Immobilized ligand before deactivation. The ligand has passed the sensor surface and most of the protein that is not covalently bound is eluted. |
| 8) | Stabilization of the double bonds by reduction with a 40 µl injection of 0.1 M cyanoborohydride in 0.1 M sodium acetate pH 4.0 at 2 µl/min. |
| 9) | Removing of remaining electrostatically bound ligand with three injection of 5 µl 10 mM HCl or Glycine at 2 µl/min. |
| 10) | Point 10 minus point 5 gives the amount of immobilized ligand after deactivation. |