This forum is intended for questions about kinetics, Surface Plasmon Resonance and the instruments related to these techniques.
Ligand Thiol Coupling
- Arnoud
- Moderator
-

Less
More
- Thank you received: 0
3 years 9 months ago - 3 years 9 months ago #7
by Arnoud
Replied by Arnoud on topic Ligand Thiol Coupling
Hi Mehraz,
Thank you for the figure. I will try to explain what you see.
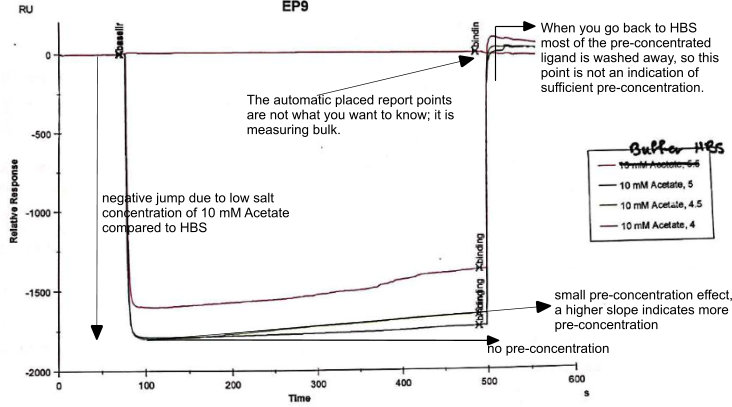
The negative jump is due to the lower salt concentration in the acetate buffer. Variations in the jump can happen due to weighing and adjusting the buffer. Next, we are looking for the pre-concentration which seems to work slightly. In general, we are looking for a slope between 10 – 50 RU/s but yours is much lower. Not sure why and it puzzles me.
One options is that such a short peptide lacks sufficient charges (despite the pI calculation) to have sufficient pre-concentration effect on a carboxylated sensor surface.
Diluting a ‘high’-pH solution in a ‘low’-pH solution has definitely some effect but is dependent on both solution strengths and dilution factor. You can try some (larger volume) dummy dilutions and check with pH-paper what the effect is. I can remember diluting a ligand in pH 3.0 just to reach pH 4.5 because of the buffer.
Thank you for the figure. I will try to explain what you see.
The negative jump is due to the lower salt concentration in the acetate buffer. Variations in the jump can happen due to weighing and adjusting the buffer. Next, we are looking for the pre-concentration which seems to work slightly. In general, we are looking for a slope between 10 – 50 RU/s but yours is much lower. Not sure why and it puzzles me.
One options is that such a short peptide lacks sufficient charges (despite the pI calculation) to have sufficient pre-concentration effect on a carboxylated sensor surface.
Diluting a ‘high’-pH solution in a ‘low’-pH solution has definitely some effect but is dependent on both solution strengths and dilution factor. You can try some (larger volume) dummy dilutions and check with pH-paper what the effect is. I can remember diluting a ligand in pH 3.0 just to reach pH 4.5 because of the buffer.
Last edit: 3 years 9 months ago by Arnoud.
Please Log in or Create an account to join the conversation.
- Mehrnaz Mehrabipour
- Topic Author
- New Member
-

Less
More
- Thank you received: 0
3 years 9 months ago #8
by Mehrnaz Mehrabipour
Replied by Mehrnaz Mehrabipour on topic Ligand Thiol Coupling
Great, thank you so much for your detailed information. It helped me a lot.
The difference between start and end of each buffer condition is 151RU in the case of 10mM acetate with a PH of 4.5. When I activated my surface with this buffer condition and immobilized my peptide, I got 279.2RU with 1080s contact time. I do not understand why it requires much longer time to immobilize.
You are right, I will also try to reduce PH to achieve the proper PH in overall.
Thank you so much,
Mehrnaz
The difference between start and end of each buffer condition is 151RU in the case of 10mM acetate with a PH of 4.5. When I activated my surface with this buffer condition and immobilized my peptide, I got 279.2RU with 1080s contact time. I do not understand why it requires much longer time to immobilize.
You are right, I will also try to reduce PH to achieve the proper PH in overall.
Thank you so much,
Mehrnaz
Please Log in or Create an account to join the conversation.
Moderators: Arnoud, Arnoud