This forum is intended for questions about kinetics, Surface Plasmon Resonance and the instruments related to these techniques.
Troubleshooting for Wave-Like Association Phase
- malayres
- Topic Author
- New Member
-

Less
More
- Thank you received: 0
4 years 2 months ago #1
by malayres
Troubleshooting for Wave-Like Association Phase was created by malayres
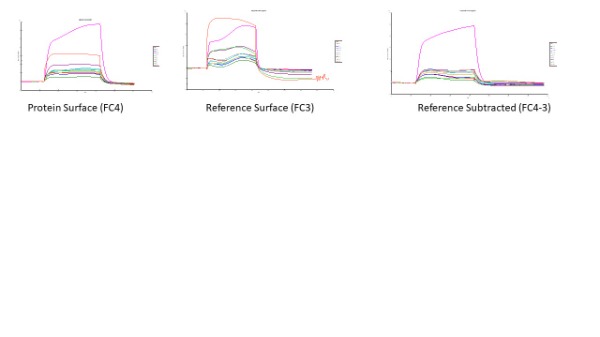
Hi. First-time post, so bear with me. I am running a protein-peptide interaction with the protein (~63kDa) immobilized on a CM5 chip surface, with reference surface blocked with ethanolamine. I am flowing the peptide (~1450 Da, 20 uL/min) over the surface at increasing concentrations for a kinetics experiment. The running buffer and sample buffer is 0.1% DMSO, 5% mannitol, 10mM HEPES in water, pH 7.4. This buffer was used based on solubility experimental results from the chemist for this particular peptide (it has very low solubility including in 10% DMSO. During the association phase of this experiment, there is a "wave" shape to some of the concentrations. I see this shape mainly on the reference surface, so reference subtraction adds to the poor sensorgram shape and response. Furthermore, I have some concentrations that create the concentration-dependent increase in RU as expected, but some concentrations (low and high) behave unexpectedly. And I ran 6 blank injections to equilibrate the system but each blank had a higher response than the previous blank and by the time my lower concentrations were analyzed, they were below baseline after blank subtraction. Does this indicate that the surface still isn't equilibrated? I have never worked with mannitol buffer before, I usually use HBS-P without issues. I am not sure what to make of these results, other than I am not comfortable reporting this data. Any suggestions from anyone how to mitigate the apparent binding-dissociation-rebinding during association phase? Or any suggestions on what is actually happening? Thanks!
Please Log in or Create an account to join the conversation.
- Arnoud
- Moderator
-

Less
More
- Thank you received: 0
4 years 2 months ago #2
by Arnoud
Replied by Arnoud on topic Troubleshooting for Wave-Like Association Phase
I don' t have a direct answer but many questions  .
.
Do the blank injections also have this "wave" in the curves? The "wave" shape can indicate that the injection system needs cleaning.
What I don't understand is that the baseline keeps going up each blank injection. How much is it per cycle and the baseline before the injection is it horizontal? Do you use any blank injections during the peptide injections? Any problems there?
Can the low solubility of the peptide be the culprit? I can imagine that it will aggregate under SPR conditions or in combination with the sensor surface (matrix and/or protein).
Kind regards
Arnoud
Do the blank injections also have this "wave" in the curves? The "wave" shape can indicate that the injection system needs cleaning.
What I don't understand is that the baseline keeps going up each blank injection. How much is it per cycle and the baseline before the injection is it horizontal? Do you use any blank injections during the peptide injections? Any problems there?
Can the low solubility of the peptide be the culprit? I can imagine that it will aggregate under SPR conditions or in combination with the sensor surface (matrix and/or protein).
Kind regards
Arnoud
Please Log in or Create an account to join the conversation.
- malayres
- Topic Author
- New Member
-

Less
More
- Thank you received: 0
4 years 2 months ago #3
by malayres
Replied by malayres on topic Troubleshooting for Wave-Like Association Phase
Hi Arnoud. Thanks for your reply! Yes, I do see it in blank injections as well. I ran a Desorb with DMSO (50%/ 10%) this afternoon and will inject some blanks to see what the baseline looks like and how many injections it takes (if ever) to return to "normal". I typically run 3 start-up cycles, then three blanks, then the increasing concentrations of peptide with no blanks once the peptide analysis starts. These are good ideas to try so that I can see what is happening there. And yes, I do agree that this is likely a multi-facet issue. Peptide solubility is definitely an issue, which is why I'm stuck with this mannitol buffer in the first place. But I think we need to do more solubility work to find something else. I'm trying to brainstorm other buffers, but not successfully. Glycerol was suggested for solubility, but from what I understand that can dissociate binding, as some use it for regeneration. Do you have any experience with using glycerol? Thanks!
Please Log in or Create an account to join the conversation.
- malayres
- Topic Author
- New Member
-

Less
More
- Thank you received: 0
4 years 2 months ago #4
by malayres
Replied by malayres on topic Troubleshooting for Wave-Like Association Phase
I'm attaching the blanks. It looks like after the 3rd cycle of blanks, they start to fall in line at the baseline prior to injection. The cycle # starts with the first cycle as the highest response, then decreases in signal following cycle number from there.
Please Log in or Create an account to join the conversation.
- Arnoud
- Moderator
-

Less
More
- Thank you received: 0
4 years 2 months ago #5
by Arnoud
Replied by Arnoud on topic Troubleshooting for Wave-Like Association Phase
A lower baseline after start-up cycles is often seen, but I don't like the wave during the injection. If start-up cycles and blank injections keep showing this wave form I would clean the system thoroughly. This means a full desorb and sanitize procedure.
The problem with glycerol is that it has a high refractive index. Kinetics is always dependent on the environment used during measurements. This means that glycerol can influence kinetics but the same is thru for higher salts or the use of detergents.
You have to find out if this is a problem. I would add one blank during the (random) concentration series and end with one blank injection to know how the system is.
Kind regards
Arnoud
The problem with glycerol is that it has a high refractive index. Kinetics is always dependent on the environment used during measurements. This means that glycerol can influence kinetics but the same is thru for higher salts or the use of detergents.
You have to find out if this is a problem. I would add one blank during the (random) concentration series and end with one blank injection to know how the system is.
Kind regards
Arnoud
Please Log in or Create an account to join the conversation.
Moderators: Arnoud, Arnoud