This forum is intended for questions about kinetics, Surface Plasmon Resonance and the instruments related to these techniques.
Trouble with immobilisation
- Raitis
- Topic Author
- New Member
-

Less
More
- Thank you received: 0
4 years 9 months ago #1
by Raitis
Trouble with immobilisation was created by Raitis
Dear all,
I have some problems with protein immobilisation. I am new to this instrument, and, unfortunately, there is no one in my institution that is deeply familiar with SPR technique, and could help me. Immobilisation was done by following the Biacore manual.
Setup and system:
1) Biacore T200 with CM7 chip;
2) The PI of the protein is ~7;
3) pH scouting suggests that pH 5.0 might be the best option (checked from pH 4.0 to 5.5); protein was in 10 mM acetate buffer with pH 5.0 (~25 ug/mL);
4) running buffer - HBS-PE (HEPES 10 mM, NaCl 150 mM, EDTA 3.4 mM, Tween-20 0.005%) + 3% DMSO (I was using the same buffer that I was planning to use for binding tests);
5) NHS/EDC (0.1 M/ 0.4 M) was prepared right before usage;
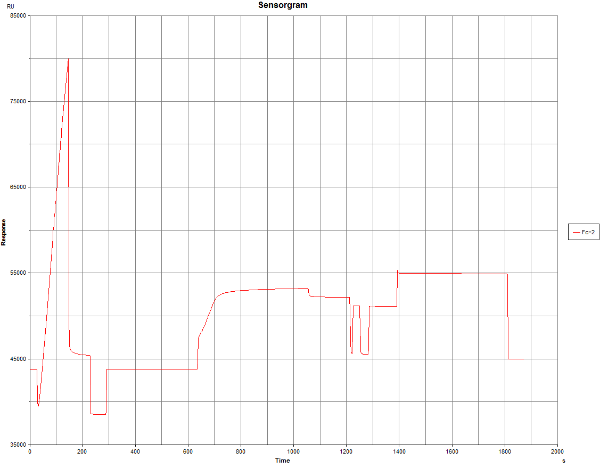
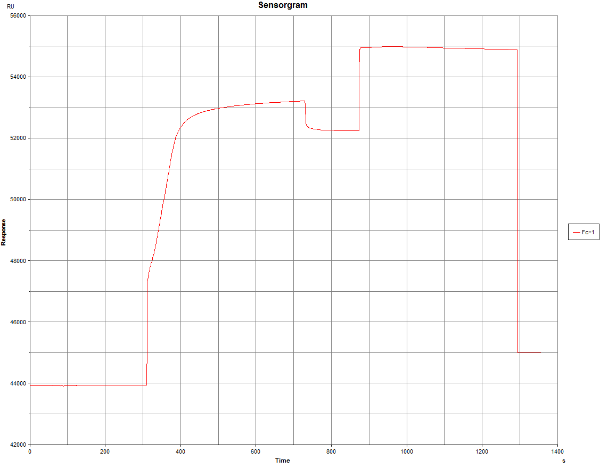
I did the first immobilisation using Biacore “Aim for 10000 RU” wizard, using default settings. The sensogramm seems to be fine at the beginning, however, there was no RU increase when the protein was added (wizard tried to add it two times). At the same run I also did prepare reference blank channel.
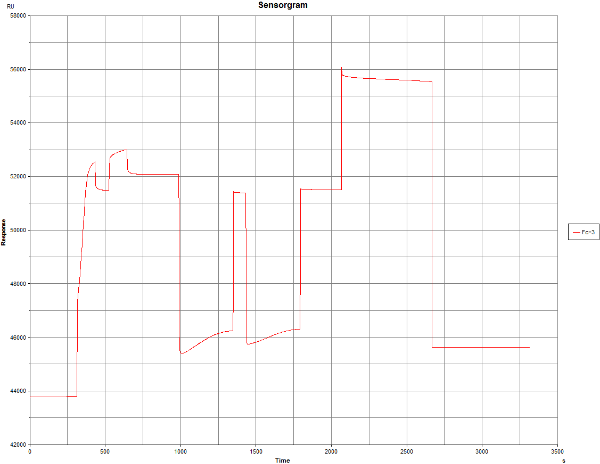
Then I tried to immobilise it manually, however, the result was similar (there is an error, where I accidently did set NHS/EDS step too short, therefore, there were two NHS/EDS injections). I did add the protein for longer time and there is some RU increase, but it is minor (both times).
Where might be the problem?
And why the RU increase after the NHS/EDC step is so high? I understand that it should be few hundreds, here it is ~8000.
And why there is really nice RU increase in pre-concentration, and no RU increase once the surface is activated?
Best regards,
Raitis
I have some problems with protein immobilisation. I am new to this instrument, and, unfortunately, there is no one in my institution that is deeply familiar with SPR technique, and could help me. Immobilisation was done by following the Biacore manual.
Setup and system:
1) Biacore T200 with CM7 chip;
2) The PI of the protein is ~7;
3) pH scouting suggests that pH 5.0 might be the best option (checked from pH 4.0 to 5.5); protein was in 10 mM acetate buffer with pH 5.0 (~25 ug/mL);
4) running buffer - HBS-PE (HEPES 10 mM, NaCl 150 mM, EDTA 3.4 mM, Tween-20 0.005%) + 3% DMSO (I was using the same buffer that I was planning to use for binding tests);
5) NHS/EDC (0.1 M/ 0.4 M) was prepared right before usage;
I did the first immobilisation using Biacore “Aim for 10000 RU” wizard, using default settings. The sensogramm seems to be fine at the beginning, however, there was no RU increase when the protein was added (wizard tried to add it two times). At the same run I also did prepare reference blank channel.
Then I tried to immobilise it manually, however, the result was similar (there is an error, where I accidently did set NHS/EDS step too short, therefore, there were two NHS/EDS injections). I did add the protein for longer time and there is some RU increase, but it is minor (both times).
Where might be the problem?
And why the RU increase after the NHS/EDC step is so high? I understand that it should be few hundreds, here it is ~8000.
And why there is really nice RU increase in pre-concentration, and no RU increase once the surface is activated?
Best regards,
Raitis
Please Log in or Create an account to join the conversation.
- Arnoud
- Moderator
-

Less
More
- Thank you received: 0
4 years 9 months ago #2
by Arnoud
Replied by Arnoud on topic Trouble with immobilisation
Hi Raitis,
In the activation part of the first sensorgram it can be seen that the curve makes a jump which is normal because of the NHS/EDC but then keeps on rising as if something is immobilising. Please compare with www.sprpages.nl/immobilization/immobilization-procedures/amine
And the same is happening with the blank immobilisation which should give only a minimal response increase.
So my guess is that something is wrong with the NHS/EDC solution. We routinely dissolve NHS and EDC separately in water and make aliquots (160 µl) in the 7mm vials + with cap which are stored at -20°C. You can store them at least one year without losing activity.
Running buffer looks fine although I would leave out the DMSO during immobilisation because of the different injection steps (analyte, NaOH, activation, deactivation are very different solutions: bulk steps) used and maybe the DMSO is interfering with the chemistry.
A note on pre-concentration before and after activation: pre-concentration if favoured by the negative surface (-COO-) but after activation there are less of these groups so pre-concentration is less effective. Normally this should not be a problem.
Kind regards
Arnoud
In the activation part of the first sensorgram it can be seen that the curve makes a jump which is normal because of the NHS/EDC but then keeps on rising as if something is immobilising. Please compare with www.sprpages.nl/immobilization/immobilization-procedures/amine
And the same is happening with the blank immobilisation which should give only a minimal response increase.
So my guess is that something is wrong with the NHS/EDC solution. We routinely dissolve NHS and EDC separately in water and make aliquots (160 µl) in the 7mm vials + with cap which are stored at -20°C. You can store them at least one year without losing activity.
Running buffer looks fine although I would leave out the DMSO during immobilisation because of the different injection steps (analyte, NaOH, activation, deactivation are very different solutions: bulk steps) used and maybe the DMSO is interfering with the chemistry.
A note on pre-concentration before and after activation: pre-concentration if favoured by the negative surface (-COO-) but after activation there are less of these groups so pre-concentration is less effective. Normally this should not be a problem.
Kind regards
Arnoud
Please Log in or Create an account to join the conversation.
- Raitis
- Topic Author
- New Member
-

Less
More
- Thank you received: 0
4 years 9 months ago #3
by Raitis
Replied by Raitis on topic Trouble with immobilisation
Thank you for the suggestion, Arnoud.
I guess, I will check the NHS/EDC reagents than.
Thank you,
Raitis
I guess, I will check the NHS/EDC reagents than.
Thank you,
Raitis
Please Log in or Create an account to join the conversation.
- Raitis
- Topic Author
- New Member
-

Less
More
- Thank you received: 0
4 years 9 months ago #4
by Raitis
Replied by Raitis on topic Trouble with immobilisation
Dear, Arnoud.
You were right - the problem was bad EDC.
The immobilisation was successful using fresh reagent.
Raitis
You were right - the problem was bad EDC.
The immobilisation was successful using fresh reagent.
Raitis
Please Log in or Create an account to join the conversation.
Moderators: Arnoud, Arnoud