This forum is intended for questions about kinetics, Surface Plasmon Resonance and the instruments related to these techniques.
Super Fast Association/Dissociation or Is There Something Else Happening?
- raulu95
- Topic Author
- New Member
-

Less
More
- Thank you received: 0
5 years 1 week ago #1
by raulu95
Super Fast Association/Dissociation or Is There Something Else Happening? was created by raulu95
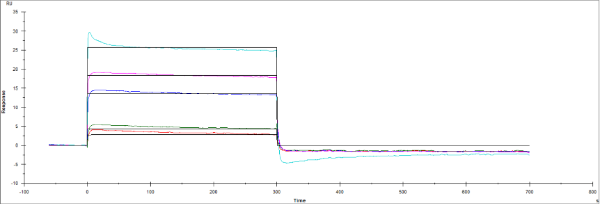
I recently ran a multi-detection experiment on the Biacore T200 where two separate ligands were immobilized onto two flow cells (CM5 carboxyl chip). The ligand I'm interested in with this questions was the second in line (it sat in low pH sodium acetate for ~3 hours). Ligand has a MW of 30 kDa and PI of 7 while analyte has a MW of 17 and pI of 5.
I immobilized about 50 ug/mL of protein and got a much lower immobilization than expected (about 200-250 RU vs. a typical immobilization of ~2000 RU). However, with that being said, I went ahead and saw rapid association and dissociation phases (no nonspecific binding to the BSA channel). Is this indicative of a bulk shift or specific phenomenon? If the ligand bound were much less active due to sitting in low pH buffer (this was the first time I did a multi-detection experiment for this specific ligand), could it exhibit an analyte response that looks like the following?
I immobilized about 50 ug/mL of protein and got a much lower immobilization than expected (about 200-250 RU vs. a typical immobilization of ~2000 RU). However, with that being said, I went ahead and saw rapid association and dissociation phases (no nonspecific binding to the BSA channel). Is this indicative of a bulk shift or specific phenomenon? If the ligand bound were much less active due to sitting in low pH buffer (this was the first time I did a multi-detection experiment for this specific ligand), could it exhibit an analyte response that looks like the following?
Please Log in or Create an account to join the conversation.
- Arnoud
- Moderator
-

Less
More
- Thank you received: 0
5 years 1 week ago #2
by Arnoud
Replied by Arnoud on topic Super Fast Association/Dissociation or Is There Something Else Happening?
Hi,
If your ligand is less active due to the low pH you expect less binding and possibly a higher non-specific interaction. The response looks ok for measurement but...
When it is real kinetics, the shape of the curves is almost totally determined by the dissociation rate. Because of the fast dissociation, the curves reach equilibrium almost directly. The height of the response is directly proportional to the analyte concentration. With increasing analyte concentration, the surface will saturate as opposed to other effects like high salt or non-specific binding.
Real kinetics can thus saturate the ligand at high analyte concentrations. If the response keeps getting higher, other effects such as non-specific binding, bulk refractive distortion causes these high responses.
So try to saturate the surface and make an equilibrium plot of the steady state versus the analyte concentration.
Arnoud
If your ligand is less active due to the low pH you expect less binding and possibly a higher non-specific interaction. The response looks ok for measurement but...
When it is real kinetics, the shape of the curves is almost totally determined by the dissociation rate. Because of the fast dissociation, the curves reach equilibrium almost directly. The height of the response is directly proportional to the analyte concentration. With increasing analyte concentration, the surface will saturate as opposed to other effects like high salt or non-specific binding.
Real kinetics can thus saturate the ligand at high analyte concentrations. If the response keeps getting higher, other effects such as non-specific binding, bulk refractive distortion causes these high responses.
So try to saturate the surface and make an equilibrium plot of the steady state versus the analyte concentration.
Arnoud
Please Log in or Create an account to join the conversation.
- raulu95
- Topic Author
- New Member
-

Less
More
- Thank you received: 0
5 years 1 week ago #3
by raulu95
Replied by raulu95 on topic Super Fast Association/Dissociation or Is There Something Else Happening?
I see what you mean. To me, it seemed like binding was on the low side because Rmax = Mw(analyte)/Mw(ligand) * immobilization level = 150 RU. To be honest, I was injecting quite a high concentration already (~10 uM where we expect a Kd of about 1 uM). It does not look like my concentrations are approaching Rmax as the maximal signal I'm obtaining here is about 30 RU. Could this indicate the ligand is less active?
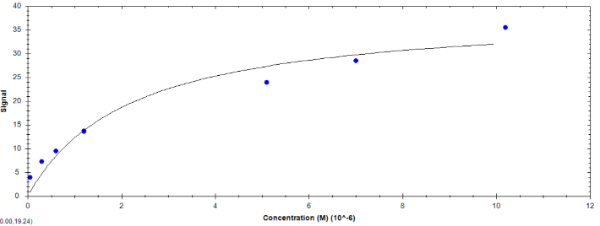
Please correct me if I'm wrong but you're essentially saying that I am not saturating the ligand so you're recommending to inject higher concentrations of analyte? I've attached an image of the steady state signal vs. concentration plate that you describe for this experiment (it gives me an estimated Kd of 2.17 uM which actually agrees with some a previous result on a different type of instrument). Is this a reliable result? Does the reason the affinity analysis seems to be stronger than kinetics in this case have to do with what you described (in terms of signal saturation) and because of the fast on and off rates?
Please correct me if I'm wrong but you're essentially saying that I am not saturating the ligand so you're recommending to inject higher concentrations of analyte? I've attached an image of the steady state signal vs. concentration plate that you describe for this experiment (it gives me an estimated Kd of 2.17 uM which actually agrees with some a previous result on a different type of instrument). Is this a reliable result? Does the reason the affinity analysis seems to be stronger than kinetics in this case have to do with what you described (in terms of signal saturation) and because of the fast on and off rates?
Please Log in or Create an account to join the conversation.
- Arnoud
- Moderator
-

Less
More
- Thank you received: 0
5 years 1 week ago - 5 years 1 week ago #4
by Arnoud
Replied by Arnoud on topic Super Fast Association/Dissociation or Is There Something Else Happening?
Don't worry about the low response. Even when you know how much ligand you have on the surface you don't know how of it is really active. So yes it could be that the ligand is less active.
To me it looks that this could be binding although the points are a little scattered (is better referencing an option?). 10 µM analyte equals about 10 times KD in your system (expected KD of 1 µM) and will give 80% of Rmax. In the end you don't have to fully saturate to have meaningful results and 10 µM seems high enough.
To get this more reliable you could immobilize a second higher (e.g. 3x) density of the ligand. Then flow the analyte over both channels and plot again the equilibrium response versus analyte concentration. If they compare you have a reliable system.
In our hands there is no difference between 1 and 2.7 µM since kinetics are dependent on many parameters such as proper analyte concentration determination and buffer composition.
(Please use KD or KD in stead of Kd.)
Arnoud
To me it looks that this could be binding although the points are a little scattered (is better referencing an option?). 10 µM analyte equals about 10 times KD in your system (expected KD of 1 µM) and will give 80% of Rmax. In the end you don't have to fully saturate to have meaningful results and 10 µM seems high enough.
To get this more reliable you could immobilize a second higher (e.g. 3x) density of the ligand. Then flow the analyte over both channels and plot again the equilibrium response versus analyte concentration. If they compare you have a reliable system.
In our hands there is no difference between 1 and 2.7 µM since kinetics are dependent on many parameters such as proper analyte concentration determination and buffer composition.
(Please use KD or KD in stead of Kd.)
Arnoud
Last edit: 5 years 1 week ago by Arnoud.
Please Log in or Create an account to join the conversation.
Moderators: Arnoud, Arnoud