This forum is intended for questions about kinetics, Surface Plasmon Resonance and the instruments related to these techniques.
about regeneration
- Wang
- Topic Author
- New Member
-

Less
More
- Thank you received: 0
7 years 11 months ago - 7 years 11 months ago #1
by Wang
about regeneration was created by Wang
hello,SPR users,
I'm a new user of Biacore.Recently I have a new problem.
my experiment : CM5 chip coupled anti human IgG Fc ,then flow antibody,and then flow antigen.
when only ligand, regeneration is oK.
when add antigen, The regeneration of high concentration analytes is incomplete.
the result is the same with different brands of antigens.
my regeneration condition is:3M MgCl2.50ul/min,30s, two regeneration!
the sensorgram as follows:
is accurate the result? How?
I'm a new user of Biacore.Recently I have a new problem.
my experiment : CM5 chip coupled anti human IgG Fc ,then flow antibody,and then flow antigen.
when only ligand, regeneration is oK.
when add antigen, The regeneration of high concentration analytes is incomplete.
the result is the same with different brands of antigens.
my regeneration condition is:3M MgCl2.50ul/min,30s, two regeneration!
the sensorgram as follows:
is accurate the result? How?
Last edit: 7 years 11 months ago by Wang.
Please Log in or Create an account to join the conversation.
- Arnoud
- Moderator
-

Less
More
- Thank you received: 0
7 years 11 months ago - 7 years 11 months ago #2
by Arnoud
Replied by Arnoud on topic about regeneration
Hi,
I have made two pictures to talk you through.
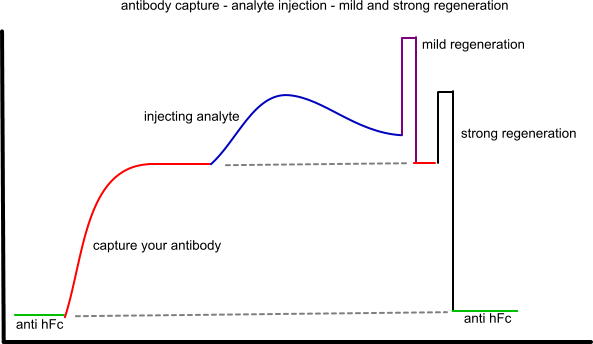
First is the sensorgram depicting the capture of your active antibody, followed by the injection of the analyte. Two regeneration steps are shown: a mild and a strong regeneration. The mild regeneration is stripping the analyte from the active antibody but is not strong enough to strip the active antibody from the anti Fc antibody. The second regeneration is strong enough to strip the active antibody from the anti Fc antibody.
This is the ideal situation, capture an antibody, do some analyte cycles (mild regeneration) en clean the surface (strong regeneration) and start over with a new antibody. Crucial is finding the proper regeneration conditions.
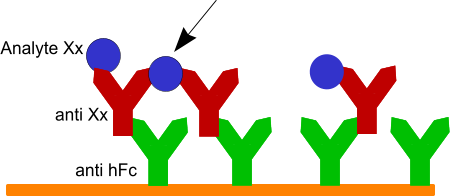
I was thinking, when the surface with the captured antibody alone (red cartoon) can be removed with the 3M MgCl2 then something changes when you add the analyte. Is it possible that there is some crosslinking between the antibodies and the analyte (arrow). This makes the interaction stronger and the surface more difficult to regenerate.
Your picture is not so clear but when you start the capture a baseline zero, it looks like that at the end after the 2 x regeneration the baseline is close to zero. If you want to use the surface for a different anti body capture and slight more stronger regeneration solution can do the trick ( www.sprpages.nl/kinetics/regeneration ).
Kind regards
Arnoud
I have made two pictures to talk you through.
First is the sensorgram depicting the capture of your active antibody, followed by the injection of the analyte. Two regeneration steps are shown: a mild and a strong regeneration. The mild regeneration is stripping the analyte from the active antibody but is not strong enough to strip the active antibody from the anti Fc antibody. The second regeneration is strong enough to strip the active antibody from the anti Fc antibody.
This is the ideal situation, capture an antibody, do some analyte cycles (mild regeneration) en clean the surface (strong regeneration) and start over with a new antibody. Crucial is finding the proper regeneration conditions.
I was thinking, when the surface with the captured antibody alone (red cartoon) can be removed with the 3M MgCl2 then something changes when you add the analyte. Is it possible that there is some crosslinking between the antibodies and the analyte (arrow). This makes the interaction stronger and the surface more difficult to regenerate.
Your picture is not so clear but when you start the capture a baseline zero, it looks like that at the end after the 2 x regeneration the baseline is close to zero. If you want to use the surface for a different anti body capture and slight more stronger regeneration solution can do the trick ( www.sprpages.nl/kinetics/regeneration ).
Kind regards
Arnoud
Last edit: 7 years 11 months ago by Arnoud.
Please Log in or Create an account to join the conversation.
- Wang
- Topic Author
- New Member
-

Less
More
- Thank you received: 0
7 years 11 months ago - 7 years 11 months ago #3
by Wang
Replied by Wang on topic about regeneration
hello,Arnoud
According to your suggestion,I changed the regeneration condition as follows:
10mM Glycine-Hcl PH1.5, flow rate: 30ul/min. contact time:35s
The regeneration is OK, but, the dissociation curve is drifting upward.
The result is not fitted by 1:1 model.What should I do next?
as follows;
Fc3 is reference.
Fc4 is active
According to your suggestion,I changed the regeneration condition as follows:
10mM Glycine-Hcl PH1.5, flow rate: 30ul/min. contact time:35s
The regeneration is OK, but, the dissociation curve is drifting upward.
The result is not fitted by 1:1 model.What should I do next?
as follows;
Fc3 is reference.
Fc4 is active
Last edit: 7 years 11 months ago by Wang.
Please Log in or Create an account to join the conversation.
- Arnoud
- Moderator
-

Less
More
- Thank you received: 0
7 years 11 months ago #4
by Arnoud
Replied by Arnoud on topic about regeneration
Hi,
pH 1.5 may be to harsh and inducing some drift as you observed.
The T200 has a method of regeneration scouting to find the best (mildest effective and giving less drift) regeneration solution. You can also use the 'cocktail'-method described in regeneration page.
It can take some experiments to find the optimal regeneration conditions.
Andersson, K. et al Identification and optimization of regeneration conditions for affinity- based biosensor assays. A multivariate cocktail approach. Analytical Chemistry 71: 2475-2481; (1999)
Arnoud
pH 1.5 may be to harsh and inducing some drift as you observed.
The T200 has a method of regeneration scouting to find the best (mildest effective and giving less drift) regeneration solution. You can also use the 'cocktail'-method described in regeneration page.
It can take some experiments to find the optimal regeneration conditions.
Andersson, K. et al Identification and optimization of regeneration conditions for affinity- based biosensor assays. A multivariate cocktail approach. Analytical Chemistry 71: 2475-2481; (1999)
Arnoud
Please Log in or Create an account to join the conversation.
- johnniewalk
- New Member
-

Less
More
- Thank you received: 0
7 years 9 months ago #5
by johnniewalk
Replied by johnniewalk on topic about regeneration
well, i think you got best answer
Please Log in or Create an account to join the conversation.
Moderators: Arnoud, Arnoud