This forum is intended for questions about kinetics, Surface Plasmon Resonance and the instruments related to these techniques.
Problems with Rmax and Fitting curves for IgG4 antibody
- Brioschi
- Topic Author
- New Member
-

Less
More
- Thank you received: 0
8 years 2 months ago #1
by Brioschi
Problems with Rmax and Fitting curves for IgG4 antibody was created by Brioschi
Hello,
I'm a new user of the Biacore T200 and since I have a really low experience I'm looking for some suggestions/explanations of some strange events I'm encountering to test the KD of two Ig4 bivalent antibodies.
I already knew that the are really strong binders and their neutralizing activity against their cognate antigen is good (tested with cellualr bioassay).
I'm actually doing a multiple cycles kinetics with the following parameters:
CHIP used: Serie S protein A
Captured antibody: 60s at 5ul/min
Sample: contact time 360s at 50ul/min
Regeneration: Glycine pH1.5 30s at 30ul/min
Dissociation time: 600s (for the Ab 1G10) 720s (for the Ab 13A1)
I diluted my antibodies in order to have around 20RUs as maximal response with the analytes concentrations.
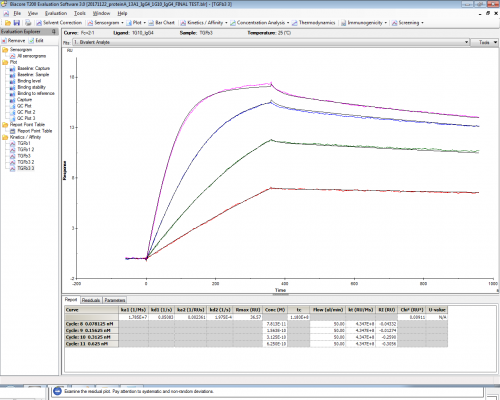
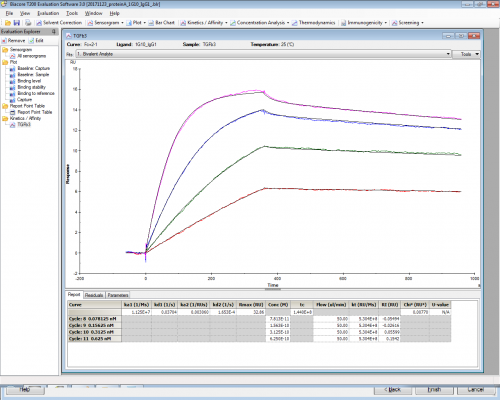
The problem is that with such a low concentration of antibody coated on the chip I cannot explain how can I reach this kind of Rmax. The theoretical Rmax should be around 3.
Atibody MW 144kDa, Analyte MW 25.8kDa
Here I attach the dissociation curves with the kinetic informations obtained with the bivalent fitting.
Antibody 1G10
Antibody 13A1
Please let me know if you need more informations.
Thank you very much
I'm a new user of the Biacore T200 and since I have a really low experience I'm looking for some suggestions/explanations of some strange events I'm encountering to test the KD of two Ig4 bivalent antibodies.
I already knew that the are really strong binders and their neutralizing activity against their cognate antigen is good (tested with cellualr bioassay).
I'm actually doing a multiple cycles kinetics with the following parameters:
CHIP used: Serie S protein A
Captured antibody: 60s at 5ul/min
Sample: contact time 360s at 50ul/min
Regeneration: Glycine pH1.5 30s at 30ul/min
Dissociation time: 600s (for the Ab 1G10) 720s (for the Ab 13A1)
I diluted my antibodies in order to have around 20RUs as maximal response with the analytes concentrations.
The problem is that with such a low concentration of antibody coated on the chip I cannot explain how can I reach this kind of Rmax. The theoretical Rmax should be around 3.
Atibody MW 144kDa, Analyte MW 25.8kDa
Here I attach the dissociation curves with the kinetic informations obtained with the bivalent fitting.
Antibody 1G10
Antibody 13A1
Please let me know if you need more informations.
Thank you very much
Please Log in or Create an account to join the conversation.
Moderators: Arnoud