This forum is intended for questions about kinetics, Surface Plasmon Resonance and the instruments related to these techniques.
response at end of injection and BiaEval software
- Arnoud
- Moderator
-

Less
More
- Thank you received: 0
7 years 5 months ago - 7 years 5 months ago #7
by Arnoud
Replied by Arnoud on topic response at end of injection and BiaEval software
Hi,
When I look at the first sensorgram, my first impresssion is that there is a lot of bulk effect in the curve. The numbers are clipped but I think it is in the order of 200 RU. You state that the buffers are matched and bulk differences should not be expected.
If this is a (double) references curve it means that the surfaces (reference and active) are not well matched and react differently to the analyte injection. You can probably see that in the raw data.
Thus the fast going down of the curve I would not see as a fast dissociation but rather a wash out of bulk salts and protein. The fitting of these curves will indeed be difficult since parts are heavily influenced by the bulk effect.
For the second sensorgram it is the same but the bulk jump looks slightly less.
One other point is that when you fit non-ideal curves, and you use only one curve (analyte concentration) the Rmax can in most cases not be determined very accurately. I recommend to inject and globally analyse at least three (better 5-6) analyte concentrations spanning 0.1 – 10x around the expected KD.
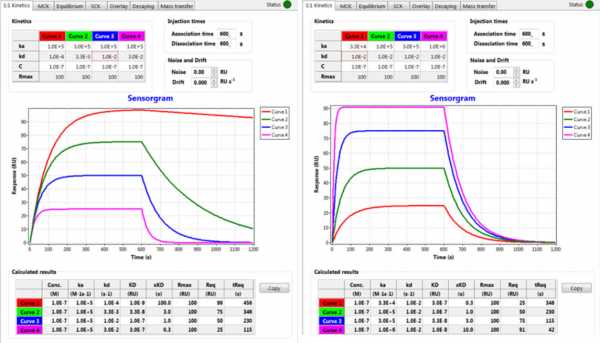
For the third sensorgram you use two different antibodies hence we expect that they have different kinetic rate constants. Since both have the same MW we expect that the binding strength is reflected in the response level (at the end of injection).
I did some modelling with SPR-Simulation ( www.sprpages.nl/spr-simulation ) to see if you assumption always hold. As you can see in the left figure where the kd is changing, you cannot assume that the highest binding level has the fastest ka since the height of the curve is determined by the kd. Indirectly the response level is determined by the analyte concentration in relation with the overall KD as can be seen in the column KD(x).
In the case that the kd is the same the response level is correlated with the ka (but look at the KD (x)).
Since the dissociation rate between the two antibodies seems not to differ too much, I can go with your assumption that a higher response has a faster ka. That said, if the bulk effect differs between the two antibodies you have a problem…
And two publication that may help you.
1. Safsten, P., Klakamp, S. L., Drake, A. W., et al.; Screening antibody-antigen interactions in parallel using Biacore A100. Analytical Biochemistry (.) 2006.
2. Shepherd, C. A., Hopkins, A. L. and Navratilova, I.; Fragment screening by SPR and advanced application to GPCRs. Progress in Biophysics and Molecular Biology (116) 2–3: 113-123; 2014.
Kind regards
Arnoud
When I look at the first sensorgram, my first impresssion is that there is a lot of bulk effect in the curve. The numbers are clipped but I think it is in the order of 200 RU. You state that the buffers are matched and bulk differences should not be expected.
If this is a (double) references curve it means that the surfaces (reference and active) are not well matched and react differently to the analyte injection. You can probably see that in the raw data.
Thus the fast going down of the curve I would not see as a fast dissociation but rather a wash out of bulk salts and protein. The fitting of these curves will indeed be difficult since parts are heavily influenced by the bulk effect.
For the second sensorgram it is the same but the bulk jump looks slightly less.
One other point is that when you fit non-ideal curves, and you use only one curve (analyte concentration) the Rmax can in most cases not be determined very accurately. I recommend to inject and globally analyse at least three (better 5-6) analyte concentrations spanning 0.1 – 10x around the expected KD.
For the third sensorgram you use two different antibodies hence we expect that they have different kinetic rate constants. Since both have the same MW we expect that the binding strength is reflected in the response level (at the end of injection).
I did some modelling with SPR-Simulation ( www.sprpages.nl/spr-simulation ) to see if you assumption always hold. As you can see in the left figure where the kd is changing, you cannot assume that the highest binding level has the fastest ka since the height of the curve is determined by the kd. Indirectly the response level is determined by the analyte concentration in relation with the overall KD as can be seen in the column KD(x).
In the case that the kd is the same the response level is correlated with the ka (but look at the KD (x)).
Since the dissociation rate between the two antibodies seems not to differ too much, I can go with your assumption that a higher response has a faster ka. That said, if the bulk effect differs between the two antibodies you have a problem…
And two publication that may help you.
1. Safsten, P., Klakamp, S. L., Drake, A. W., et al.; Screening antibody-antigen interactions in parallel using Biacore A100. Analytical Biochemistry (.) 2006.
2. Shepherd, C. A., Hopkins, A. L. and Navratilova, I.; Fragment screening by SPR and advanced application to GPCRs. Progress in Biophysics and Molecular Biology (116) 2–3: 113-123; 2014.
Kind regards
Arnoud
Last edit: 7 years 5 months ago by Arnoud. Reason: typos
Please Log in or Create an account to join the conversation.
- BIAAU
- Topic Author
- New Member
-

Less
More
- Thank you received: 0
7 years 5 months ago - 7 years 5 months ago #8
by BIAAU
Hi I really appreciate your feedback, as no one in our institute is an expert at Biacore and I am still learning!!
I have attached some files so you can see the RI response, and see how the graphs look if there is anything wrong with them.
the first files shows what buffer looks like run over FC1 as well as what the highest concentration (i run 5) of my sample looks also over Fc1 (uncoated)
The bit at the end is the regeneration injection
so the bulk response of HBS buffer or my analyte is the same, no non specific binding really and it looks when i y transform about 200RU is this normal?
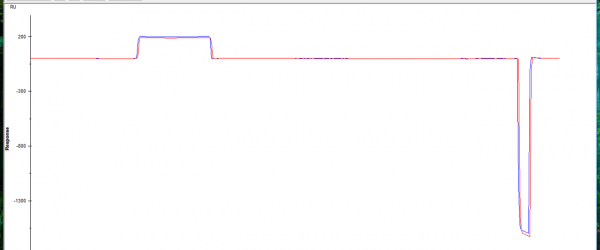
The HBS buffer only is a flat line after refrencing (inserted in lower right "example pic" although i have a spike at the start always not sure if its an instrument problem? but it is flat
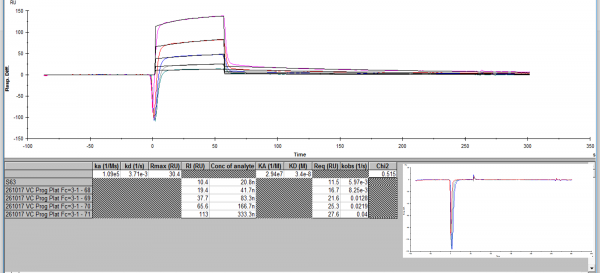
my FC2-1 (coated vs uncoated) subtracts this bulk response of 200RU to give me about 300RU in the example picture (it's 500RU on just Fc2)
I run from low to high conc. from what i have read the RI should be low, but it is 113 at the highest analyte conc? :dry:
the RI is never low regardless of the analyte.
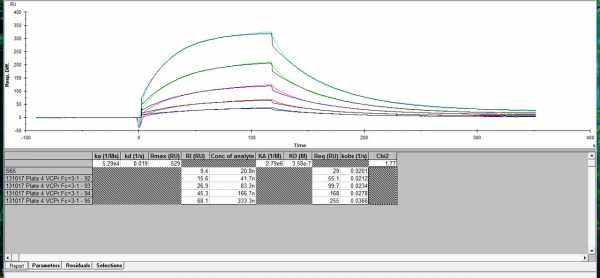
I have added same analyte diffrent receptor example2
many manay thanks
Replied by BIAAU on topic response at end of injection and BiaEval software
Hi I really appreciate your feedback, as no one in our institute is an expert at Biacore and I am still learning!!
I have attached some files so you can see the RI response, and see how the graphs look if there is anything wrong with them.
the first files shows what buffer looks like run over FC1 as well as what the highest concentration (i run 5) of my sample looks also over Fc1 (uncoated)
The bit at the end is the regeneration injection
so the bulk response of HBS buffer or my analyte is the same, no non specific binding really and it looks when i y transform about 200RU is this normal?
The HBS buffer only is a flat line after refrencing (inserted in lower right "example pic" although i have a spike at the start always not sure if its an instrument problem? but it is flat
my FC2-1 (coated vs uncoated) subtracts this bulk response of 200RU to give me about 300RU in the example picture (it's 500RU on just Fc2)
I run from low to high conc. from what i have read the RI should be low, but it is 113 at the highest analyte conc? :dry:
the RI is never low regardless of the analyte.
I have added same analyte diffrent receptor example2
many manay thanks
Last edit: 7 years 5 months ago by BIAAU. Reason: typos
Please Log in or Create an account to join the conversation.
- Arnoud
- Moderator
-

Less
More
- Thank you received: 0
7 years 5 months ago #9
by Arnoud
Replied by Arnoud on topic response at end of injection and BiaEval software
Hi,
When you inject flow buffer over the sensor surfaces (both reference and active) you expect a more or less flat (± 5-15 RU) injection line. A bulk of 200 RU upon injection of flow buffer is not acceptable. Let's make things sure, with an injection a flow buffer I mean that you take some buffer from the buffer compartment and put it in a vial and inject it right away. Did you do that?
If so and you get 200 RU of bulk something is wrong and my suggestion would be to run a desorb / sanitize and wash overnight with fresh filtered and degassed running buffer.
The other thing you have to be aware of is that the four channels are in series. Thus the buffer arrives at the first channel slightly earlier than at the second, etcetera. The delay is dependent on the flow rate. Thus before you can do reference subtraction you have to x-align the curves. Failing to do so can explain the negative spike in the small inserted picture.
One other possibility is that the injection port / needle is not clean and gives some injection disturbances.
The last picture looks way better than you posted before. When you can reduce the bulk jumps, the fitting will be even better.
You can also do the fitting with the RI parameter set to constant 0. Normally we first fit the dissociation alone to get an idea of the off rate, then we fit the association and dissociation (the dissociation should not change too much) and in the last round we add the RI.
In addition, if possible you can immobilize a little less receptor so the overall response gets lower. A lower response is better. I would go for a maximum response of less than 100 RU.
Maybe you should also take a look (and buy) at the SPR pages book ( www.sprpages.nl/contact-spr-pages/sprpages-book ). It is a valuable companion besides your instrument and it contains more tips and tricks than the website.
Regards
Arnoud
When you inject flow buffer over the sensor surfaces (both reference and active) you expect a more or less flat (± 5-15 RU) injection line. A bulk of 200 RU upon injection of flow buffer is not acceptable. Let's make things sure, with an injection a flow buffer I mean that you take some buffer from the buffer compartment and put it in a vial and inject it right away. Did you do that?
If so and you get 200 RU of bulk something is wrong and my suggestion would be to run a desorb / sanitize and wash overnight with fresh filtered and degassed running buffer.
The other thing you have to be aware of is that the four channels are in series. Thus the buffer arrives at the first channel slightly earlier than at the second, etcetera. The delay is dependent on the flow rate. Thus before you can do reference subtraction you have to x-align the curves. Failing to do so can explain the negative spike in the small inserted picture.
One other possibility is that the injection port / needle is not clean and gives some injection disturbances.
The last picture looks way better than you posted before. When you can reduce the bulk jumps, the fitting will be even better.
You can also do the fitting with the RI parameter set to constant 0. Normally we first fit the dissociation alone to get an idea of the off rate, then we fit the association and dissociation (the dissociation should not change too much) and in the last round we add the RI.
In addition, if possible you can immobilize a little less receptor so the overall response gets lower. A lower response is better. I would go for a maximum response of less than 100 RU.
Maybe you should also take a look (and buy) at the SPR pages book ( www.sprpages.nl/contact-spr-pages/sprpages-book ). It is a valuable companion besides your instrument and it contains more tips and tricks than the website.
Regards
Arnoud
Please Log in or Create an account to join the conversation.
- BIAAU
- Topic Author
- New Member
-

Less
More
- Thank you received: 0
7 years 5 months ago #10
by BIAAU
Replied by BIAAU on topic response at end of injection and BiaEval software
Thanks for your reply
I contacted my local Biacore rep who suggested a few things aswell.
I followed your suggestions too.
I've also exchanged the needle to no affect.
Still large bulk response injecting buffer from buffer compartment into buffer. This is HBS-EP which we buy from GE
The bulk actually increased a little after sanitize
Any other suggestions? the service will cost over $10,000 which we cant justify for such an old machine. Any DIY ideas
would running concentrated sodium hypo for the sanitize procedure have caused the problem (it is supposed to be diluted significantly) as someone who used it before may have run full conc sanitize.
I dont know if you are familar with the Biacore3000 but the screw is quite rusty around the injection port, i washed these with water, but no change.
any help would be great, as i dont want to give up on the machine just yet!
I think the data should be ok after all the bulk is subtracted.
Many thanks again
I contacted my local Biacore rep who suggested a few things aswell.
I followed your suggestions too.
I've also exchanged the needle to no affect.
Still large bulk response injecting buffer from buffer compartment into buffer. This is HBS-EP which we buy from GE
The bulk actually increased a little after sanitize
Any other suggestions? the service will cost over $10,000 which we cant justify for such an old machine. Any DIY ideas
would running concentrated sodium hypo for the sanitize procedure have caused the problem (it is supposed to be diluted significantly) as someone who used it before may have run full conc sanitize.
I dont know if you are familar with the Biacore3000 but the screw is quite rusty around the injection port, i washed these with water, but no change.
any help would be great, as i dont want to give up on the machine just yet!
I think the data should be ok after all the bulk is subtracted.
Many thanks again
Please Log in or Create an account to join the conversation.
- Arnoud
- Moderator
-

Less
More
- Thank you received: 0
7 years 5 months ago #11
by Arnoud
Replied by Arnoud on topic response at end of injection and BiaEval software
Using a high sodium hypochlorite is not recommended but it should not cause high bulk upon buffer injection. The cause must be somewhere in the injection / flow system. The instrument is properly cleaned and sanitised thus the tubing and the IFC should be clean. You can take the injection port out (unscrew the rusty screw and detach the flow tubing. Wash it under the tap and make it dry. Check if the injection port (the cup where the needle goes in) is firmly secured and free of salts and scratches on the inside.
Mount the injection block (reattach the flow line properly!) and do a needle replacement routine to check that the needle is properly going into the injection port.
The needle should go down and press for 1 mm into the port (the spring on top of the needle goes up 1 mm).
Check the pumps for air and leakage.
Dock a new sensor chip and equilibrate thoroughly (3x prime and flow until all lines are horizontal).
If possible, normalize the system with the glycerol solution from the maintenance kit.
Prime two times after normalization.
Inject some flow buffer. What do you see?
In addition, you can do a full system check to check for valve errors etc.
If nothing comes up I don't have any good idea's any more.
If the bulk jump is the same in both reference and active channel and reference subtraction is acceptable, probably you have to live with this quirk of your system.
Kind regards
Arnoud
Mount the injection block (reattach the flow line properly!) and do a needle replacement routine to check that the needle is properly going into the injection port.
The needle should go down and press for 1 mm into the port (the spring on top of the needle goes up 1 mm).
Check the pumps for air and leakage.
Dock a new sensor chip and equilibrate thoroughly (3x prime and flow until all lines are horizontal).
If possible, normalize the system with the glycerol solution from the maintenance kit.
Prime two times after normalization.
Inject some flow buffer. What do you see?
In addition, you can do a full system check to check for valve errors etc.
If nothing comes up I don't have any good idea's any more.
If the bulk jump is the same in both reference and active channel and reference subtraction is acceptable, probably you have to live with this quirk of your system.
Kind regards
Arnoud
Please Log in or Create an account to join the conversation.
- BIAAU
- Topic Author
- New Member
-

Less
More
- Thank you received: 0
7 years 5 months ago #12
by BIAAU
Replied by BIAAU on topic response at end of injection and BiaEval software
Hi figured out the cause of the bulk.
I had a vial of the buffer (HBS-EP) i kept using but when i used freshly opened buffer i so no bulk (less than 5 RU) but with buffer that had been exposed to air i got 200RU, the longer i left it sitting the higher bulk response.
I didn;t get this with the buffer i capped and left sitting out.
Curious, have you ever seen this? also a plastic vial gave higher bulk of buffer that glass
Thanks for all your help!!!!!!!!!!
I had a vial of the buffer (HBS-EP) i kept using but when i used freshly opened buffer i so no bulk (less than 5 RU) but with buffer that had been exposed to air i got 200RU, the longer i left it sitting the higher bulk response.
I didn;t get this with the buffer i capped and left sitting out.
Curious, have you ever seen this? also a plastic vial gave higher bulk of buffer that glass
Thanks for all your help!!!!!!!!!!
Please Log in or Create an account to join the conversation.
Moderators: Arnoud, Arnoud