This forum is intended for questions about kinetics, Surface Plasmon Resonance and the instruments related to these techniques.
pH scouting - No complete dissociation.
- Layzing
- Topic Author
- New Member
-

Less
More
- Thank you received: 0
9 years 10 months ago - 9 years 10 months ago #1
by Layzing
pH scouting - No complete dissociation. was created by Layzing
Hi there.
Machine: Reichert SR7000dc
Chip: Xantec polycarboxylated hydrogel (HC) 200M.
Protein solution - 5 mM sodium acetate pH 4-5 + 25 µg/mL IgG antibody (pI 6.4-9.0 ?)
Running buffer - degassed DI water
Cleaning solution
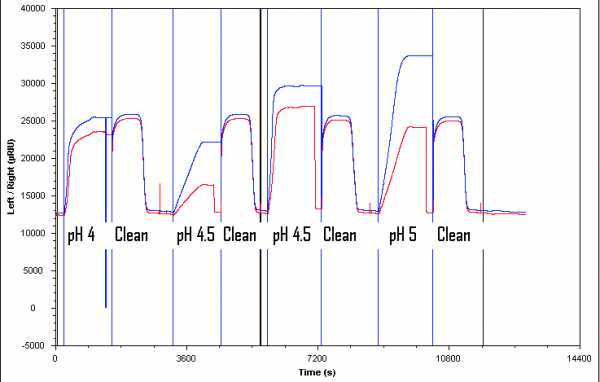
I've seen from other pH scoutings that the measurement return immediately to baseline after the protein solution injection ends. In my case, this doesn't happend. I've read that this suggest too low pH and denaturation of protein. Is this the case in my experiment, or what do you think? And what would you do to solve it?
Also, upon 'pump refill', it returns to baseline in right channel (red) even before cleaning solution injection.
Best regards,
Layzing
Machine: Reichert SR7000dc
Chip: Xantec polycarboxylated hydrogel (HC) 200M.
Protein solution - 5 mM sodium acetate pH 4-5 + 25 µg/mL IgG antibody (pI 6.4-9.0 ?)
Running buffer - degassed DI water
Cleaning solution
I've seen from other pH scoutings that the measurement return immediately to baseline after the protein solution injection ends. In my case, this doesn't happend. I've read that this suggest too low pH and denaturation of protein. Is this the case in my experiment, or what do you think? And what would you do to solve it?
Also, upon 'pump refill', it returns to baseline in right channel (red) even before cleaning solution injection.
Best regards,
Layzing
Last edit: 9 years 10 months ago by Layzing.
Please Log in or Create an account to join the conversation.
- Arnoud
- Moderator
-

Less
More
- Thank you received: 0
9 years 10 months ago #2
by Arnoud
Replied by Arnoud on topic pH scouting - No complete dissociation.
Hi Layzing,
Because you use an antibody I would recommend to use a buffer instead of DI water. A buffer (Hepes, Tris) with 150 mM NaCl will lower electrostatic interactions with the matrix and maybe the baseline will return.
Do you know the content of the cleaning solution? In Biacore 50 mM NaOH is used to clean the chip before immobilization. It should be compatible with the Xantec HC chip.
Arnoud
Because you use an antibody I would recommend to use a buffer instead of DI water. A buffer (Hepes, Tris) with 150 mM NaCl will lower electrostatic interactions with the matrix and maybe the baseline will return.
Do you know the content of the cleaning solution? In Biacore 50 mM NaOH is used to clean the chip before immobilization. It should be compatible with the Xantec HC chip.
Arnoud
Please Log in or Create an account to join the conversation.
- Layzing
- Topic Author
- New Member
-

Less
More
- Thank you received: 0
9 years 10 months ago #3
by Layzing
Replied by Layzing on topic pH scouting - No complete dissociation.
Hi Arnoud.
Thank you for the reply.
During the pH scouting I swapped to PBS buffer as the running buffer, and as you imply the measurements now return to baseline after injection. You think I should use PBS running buffer under immobilizatio, despite Xantec immobilization protocol states that immobilization should be performed with dd water as running buffer?
Yes, the cleaning/elution buffer consist of 1 M sodium chloride in 0.1 M sodium borate pH 9.0, but it seems to work just fine as is.
I am currently working on my master about kinetics of GLP-1 and various GLP-1 antibodies interactions and epitope mapping. I am not the most experienced with SPR, so I appreciate all the help you can provide. Thanks for now, hopefully you'll be willing to assist me upon future complications.
Best regards,
Lasse
Thank you for the reply.
During the pH scouting I swapped to PBS buffer as the running buffer, and as you imply the measurements now return to baseline after injection. You think I should use PBS running buffer under immobilizatio, despite Xantec immobilization protocol states that immobilization should be performed with dd water as running buffer?
Yes, the cleaning/elution buffer consist of 1 M sodium chloride in 0.1 M sodium borate pH 9.0, but it seems to work just fine as is.
I am currently working on my master about kinetics of GLP-1 and various GLP-1 antibodies interactions and epitope mapping. I am not the most experienced with SPR, so I appreciate all the help you can provide. Thanks for now, hopefully you'll be willing to assist me upon future complications.
Best regards,
Lasse
Please Log in or Create an account to join the conversation.
Moderators: Arnoud, Arnoud