This forum is intended for questions about kinetics, Surface Plasmon Resonance and the instruments related to these techniques.
The shape of sensorgrams
- VesnaH
- Topic Author
- New Member
-

Less
More
- Thank you received: 0
10 years 10 months ago - 10 years 10 months ago #1
by VesnaH
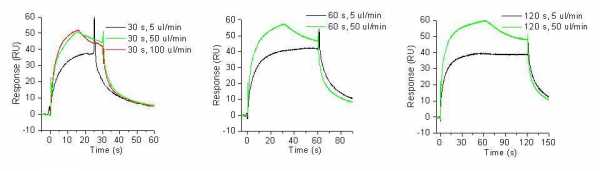
I am studying the binding of small analytes to the protein. With several different ligands I've noticed that the curves have a "step" in the middle of the association phase. When changing to slower flow rate, the sensorgrams looks normal, but I am affraid that in this case I am working under mass transfer limitations.
Any comments?
The shape of sensorgrams was created by VesnaH
I am studying the binding of small analytes to the protein. With several different ligands I've noticed that the curves have a "step" in the middle of the association phase. When changing to slower flow rate, the sensorgrams looks normal, but I am affraid that in this case I am working under mass transfer limitations.
Any comments?
Last edit: 10 years 10 months ago by VesnaH.
Please Log in or Create an account to join the conversation.
- Arnoud
- Moderator
-

Less
More
- Thank you received: 0
10 years 9 months ago #2
by Arnoud
Replied by Arnoud on topic The shape of sensorgrams
When the response is dropping during the analyte injection, the cause can be of several origins.
1. Mixing of sample and buffer
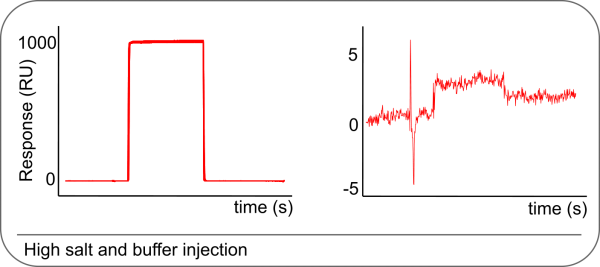
When the response during the analyte injection is dropping, it can indicate that there is sample dispersion. The sample is mixing with the flow buffer, resulting in an effective lower analyte concentration (1). Most SPR machines have special routines to separate the flow buffer from the sample. Use them and check if the sample is properly separated from the flow buffer.
Problems with sample dispersion are easily recognized by injecting an elevated NaCl solution (0.5 M). The NaCl solution must give a sharp rise and fall when injected and have a flat steady state. In addition, visually inspect the separation of the sample and flow buffer by the air bubbles. If the separation is not clear, clean the tubing with 0.5% SDS and 50 mM NaOH pH 9.5. Wash through with running buffer and check again.
2. Competition between two variants (size and affinity)
When the analyte contains several variants, which differ in size and affinity, the higher affinity variant will displace the lower affinity variant. If the higher affinity variant has a distinct lower molecular weight, the response drops during the displacement. Check the analyte for purity by gel electrophoresis.
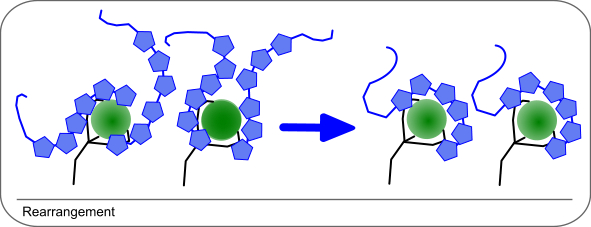
3. Rearrangement of surface
In some cases it is assumed that the initial interaction if flooding the ligand. Therefore, the interaction is not 1:1 but more than one analyte molecule is interacting with the ligand. During the injection, the stronger bound analyte displaces the weaker bound analyte, which has the effect that less mass is bound to the sensor chip. Hence, the response is lowering during injection.
When lower analyte concentrations are injected, this effect is less and the binding curve becomes 1:1 again.
4. Reference channel is not good
When the reference channel is binding more than the ligand, then after subtracting, the ligand binding is lower. This can result in negative responses in the extreme cases or a decrease in response during the injection. Check the raw data of ligand and reference channel before subtracting. Ideally, the reference should have a low response (< 10% of ligand signal) and a normal curve without disturbances.
1. Rich, R. L. and Myszka, D. G.; Survey of the year 2001 commercial optical biosensor literature. J.Mol.Recognit. (15): 352-376; 2002.
1. Mixing of sample and buffer
When the response during the analyte injection is dropping, it can indicate that there is sample dispersion. The sample is mixing with the flow buffer, resulting in an effective lower analyte concentration (1). Most SPR machines have special routines to separate the flow buffer from the sample. Use them and check if the sample is properly separated from the flow buffer.
Problems with sample dispersion are easily recognized by injecting an elevated NaCl solution (0.5 M). The NaCl solution must give a sharp rise and fall when injected and have a flat steady state. In addition, visually inspect the separation of the sample and flow buffer by the air bubbles. If the separation is not clear, clean the tubing with 0.5% SDS and 50 mM NaOH pH 9.5. Wash through with running buffer and check again.
2. Competition between two variants (size and affinity)
When the analyte contains several variants, which differ in size and affinity, the higher affinity variant will displace the lower affinity variant. If the higher affinity variant has a distinct lower molecular weight, the response drops during the displacement. Check the analyte for purity by gel electrophoresis.
3. Rearrangement of surface
In some cases it is assumed that the initial interaction if flooding the ligand. Therefore, the interaction is not 1:1 but more than one analyte molecule is interacting with the ligand. During the injection, the stronger bound analyte displaces the weaker bound analyte, which has the effect that less mass is bound to the sensor chip. Hence, the response is lowering during injection.
When lower analyte concentrations are injected, this effect is less and the binding curve becomes 1:1 again.
4. Reference channel is not good
When the reference channel is binding more than the ligand, then after subtracting, the ligand binding is lower. This can result in negative responses in the extreme cases or a decrease in response during the injection. Check the raw data of ligand and reference channel before subtracting. Ideally, the reference should have a low response (< 10% of ligand signal) and a normal curve without disturbances.
1. Rich, R. L. and Myszka, D. G.; Survey of the year 2001 commercial optical biosensor literature. J.Mol.Recognit. (15): 352-376; 2002.
Please Log in or Create an account to join the conversation.
- VesnaH
- Topic Author
- New Member
-

Less
More
- Thank you received: 0
10 years 9 months ago #3
by VesnaH
Replied by VesnaH on topic The shape of sensorgrams
Hi Arnoud, thx for the reply.
It could be that we have some problems with the fluidics, cause I've observed the similar problems with different projects. And the problem is always more obvious at higher flow rates. We clean the microfuidics regularly, but it could be that this is not enough anymore and it needs to be exchanged.
I can not influence on the injection of the samples (i'm usng Biacore T100, and always the option "High performance" for the sample injection).
All best,
Vesna
It could be that we have some problems with the fluidics, cause I've observed the similar problems with different projects. And the problem is always more obvious at higher flow rates. We clean the microfuidics regularly, but it could be that this is not enough anymore and it needs to be exchanged.
I can not influence on the injection of the samples (i'm usng Biacore T100, and always the option "High performance" for the sample injection).
All best,
Vesna
Please Log in or Create an account to join the conversation.
Moderators: Arnoud, Arnoud